Cancer Diagnostic Probe




The Efficiency of Cancer Diagnostic Probe in Breast Cancer
Directly checking the margins of the cavity after tumor excision can help prevent any remaining tumor residues, satellite, or scattered cancer cells. Published reports have indicated that conventional intraoperative methods, such as frozen section and X-ray evaluation of dissected tumor margins, still miss diagnosing more than 20% of the involved margins. It is also important to remove all the involved lymph nodes from the body to reduce the risk of local recurrences. The Cancer Diagnostic Probe (CDP) is a real-time diagnostic system that serves as a complementary surgeon-assisted tool. It is used in conjunction with frozen-section and permanent pathologies to detect high-risk pre-cancer/cancer cells in the cavity side margins and cancerous cells in the lymph nodes of patients undergoing breast cancer surgery. In modern cancer therapy, these probes play a crucial role due to their precision and ability to provide detailed information about the presence and characteristics of cancer. This ultimately leads to improved diagnosis, treatment, and patient outcomes.Scientific Advisors
Description
Checking the cavity-side margins during surgery for breast cancer patients is critical to ensure the definitive removal of suspicious and high-risk lesions with minimal damage to normal tissue. Remaining cancer cells in the breast causes re-surgeries and inevitable post-surgical treatments, which will have several side effects. Frozen pathology of tumor margins during surgery is a clinically accepted procedure to guide the surgeon to any need for re-excision. However, the time-consuming and expert dependent process of margin examination and false diagnostics, especially in neoadjuvant cases, are limitations of this procedure. CDP ( Cancer Diagnostic Probe ) system, has been introduced as a surgical assistant system in breast cancer surgery. The new system reveals the cancerous cells in the internal margins (cavity-side margin) within a few millimeters in 40 seconds, using a needle sensor. This system has a clinical diagnostic classification matching with the pathological results of the tested tissues. The CDP response peaks are based on the classification of the pathological system (Ductal intraepithelial neoplasia (DIN), Lobular intraepithelial neoplasia (LIN), and Fibro epithelial lesion (FEL) (according to the latest reported revisions)). The distinctive ability of CDP to detect cancer cells in the internal margins (after tumor dissection) has the sensitivity and selectivity of 97% and 94%, respectively.CDP Testimonials
-

-
Dr. Seyed Rouholah Miri
Assistant Professor of Surgery at Tehran & Alborz University of Medical Sciences
Technical Data
CDP ( Cancer Diagnostic Probe ) in the Lymph Node Mode Involved lymph node detection is also performed based on measuring the impedimetric properties associated with Fatty Acid Oxidation (FAO), which is the most dominant cancer cell metabolism in the lymph node environment.Hospitech Cancer Diagnostic Probe Standards
- IEC 60601-1: 2016: international Standard: General requirements for basic safety and essential performance for medical equipment.
- IEC 60601-1-2: 2014: EMC Compliance, General requirements for basic safety and essential performance - Collateral Standard: Electromagnetic disturbances- Requirements and tests.
- ISO 62304: Medical Device software- Life cycle Process.
- ISO 10993-10: Biological evaluation of medical devices: Part 10: test for irritation and skin sensitization.
- ISO 10993-5: Biological evaluation of medical devices: Part 5: test for in- vitro cytotoxicity .
- ISO 11607-1: Packaging for terminally sterilized medical devices
- PART1: requirement for materials, sterile barrier systems, and packaging systems. INSO 3001-1: Sterility compliance
- ISO 13485: Medical Devices- Quality management systems.
Hospitech Cancer Diagnostic Probe
Hospitech Cancer Care Innovations Company is leading the way in revolutionizing cancer diagnosis with its innovative range of devices. These include the Cancer Diagnostic Probe (CDP), Impedimetric Tumor Detection System (ITDS), Electrical Endoscopy Mass Detection (EMD), and ElectroChemoTherapy (ECT) & Gamma Probe. These cutting-edge technologies are designed to improve cancer detection and treatment efficacy. Supported by research, articles, and patents, these tools provide accurate results for early diagnosis and better patient outcomes. The company takes pride in its dedicated team and unwavering commitment to making a significant impact in the healthcare industry's fight against cancer. Upgrade your medical center by utilizing Hospital Tech's breast cancer diagnostic product and benefit from an innovative and safe approach to breast tumor surgery and treatment.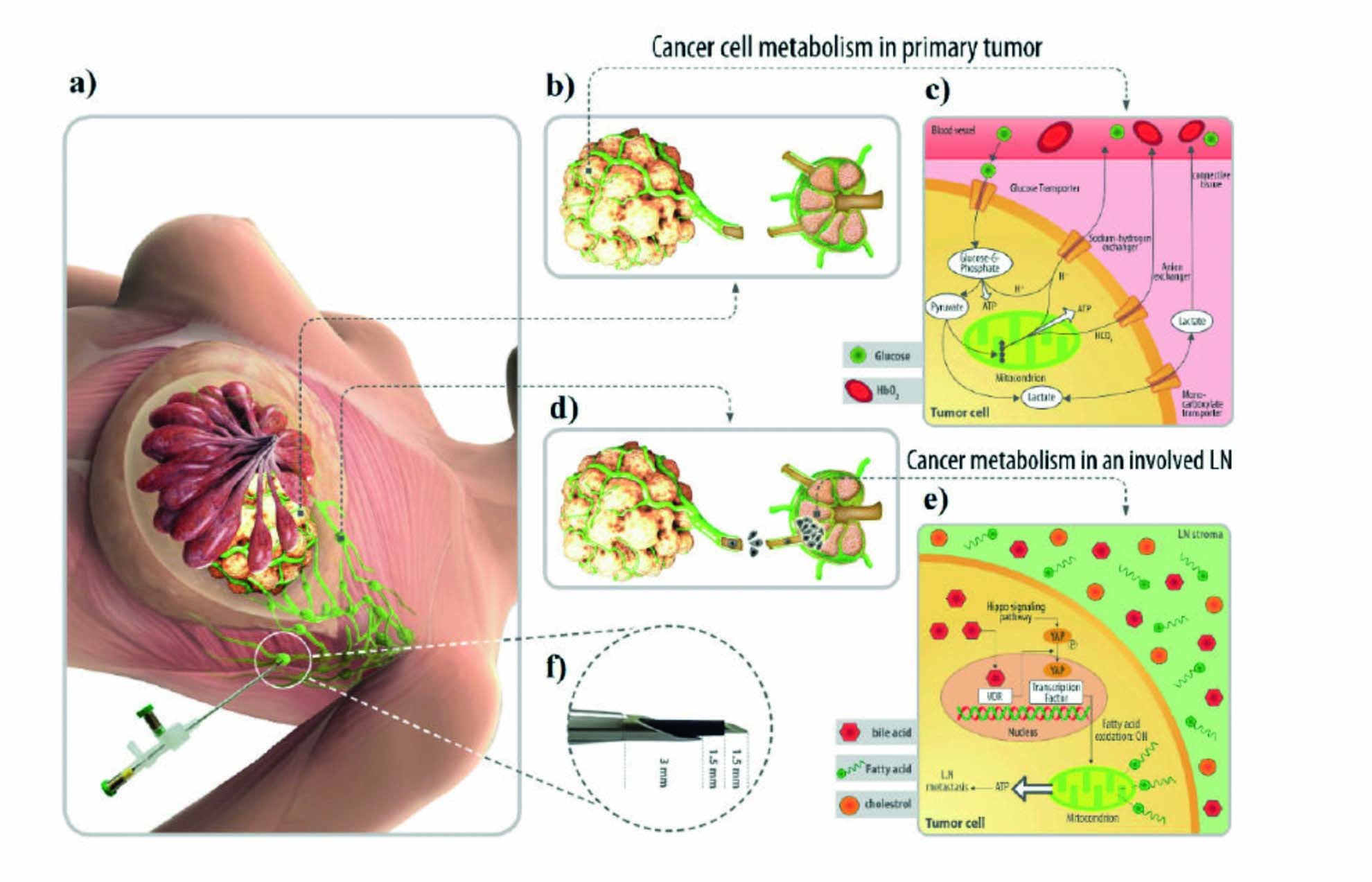
- Applying an LN headprobe on an auxiliary lymph node to detect LN involvement
- Hematic view of a lymph node free of cancerous cells
- The metabolism of cancer cells in the primary tumor site based on taking glucose from blood vessels (hypoxia glycolysis)
- Schematic view of cancer cells spreading from the primary tumor site and invasion to a lymph node environment through lymphatic vessels
- With the metabolic shift of cancer cells to Fatty Acid Oxidation (FAO)
- Dimensions of an LN headprobe


Mohammad Abdolahad
Mohammad Abdolahad was born in Tehran, Iran, in 1982. He received his Ph.D. in Nanoelectronic Engineering from the University of Tehran in 2013. Immediately, he started teaching as an academic staff member in the College of Engineering, the School of Electrical and Computer Engineering. In the last few years, he has published more than 70 articles. He has 45 US patents in the field of cancer diagnosis and treatment devices ( Cancer Diagnostic Probe ). He has won many honors, including the Mustafa Award (2019), Selected Researcher of the Country (2019), Selected Young Researcher in 2017, etc.CDP Articles
Case Reports
Read More Case ReportsUS Patents
Iran IMED
Inida DCGI
Trial Phase

Oman MOH
Under Process

A cancer diagnostic probe offers advanced features, including high sensitivity for early detection and precision in identifying tumor margins. This technology has the potential to improve patient prognosis by enabling early intervention and reducing the need for additional surgeries, thus minimizing patient discomfort and treatment costs. Additional features of this device include the following:
Technology of Cancer Diagnostic Probe
- CDP in the margin mode
CDP›s detection mechanism in the margin mode has been based on real-time detection of released ROS/H2O, molecules by cancer cells during tumor initiation, reverse Warburg effect, and hypoxia-assisted glycolysis.
- CDP in the lymph node mode
Involved lymph node detection is also performed based on measuring the impedimetric properties associated with Faxy Acid Oxidation (FAO), which is the most dominant cancer cell metabolism in the lymph node environment.
In the cancer diagnostic probe device, we have incorporated the latest and most accurate techniques to detect and identify molecules released from cancer cells for real-time diagnosis with minimal margin of error. This identification process is carried out in two ways:
Features of Cancer Diagnostic Probe
- Real-time and non-invasive detection of involved cavity side margins which are not detected by frozen-section pathology
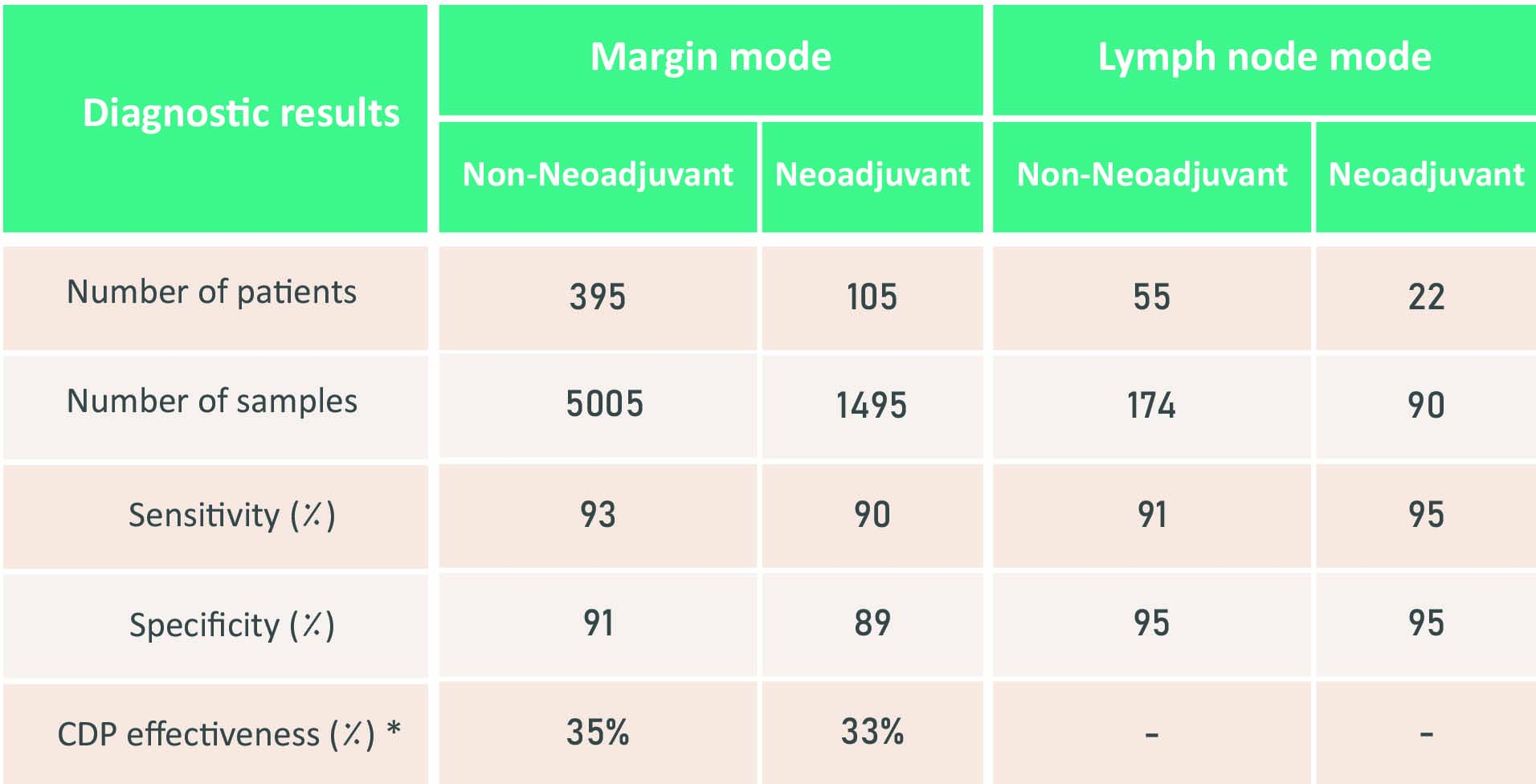
- A diagnostic accuracy of over 93% during breast cancer surgery
- Real-time detection of involved lymph nodes with 91% sensitivity
- Reduction of about 30% of the involved cavity side margins
- Intraoperative diagnosis of excision-required newly discovered solid masses that were not evaluated in presurgical evaluations
- Increasing the prognosis factor and reducing the local recurrence rate in breast cancer patients.
- Using disposable head probes to prevent the transmission of contamination
FAQ

Related products
Gamma Probe
Thyroid Nodule Impedance Measurement System (TN_IMS)
QUICK LINKS
NEWSLETTER










































